Harmonizing innovation, bridging the future | EverBridge shines at OCIN 2024, leading a new chapter in the innovation and implementation of neurointerventional devices
Updates
2024.11.06
39
From October 24 to 27, 2024, the Oriental Conference on Cerebrovascular Diseases 2024 (OCIN 2024) and the Academic Annual Meeting of the Stroke Branch of the Shanghai Medical Association successfully concluded in Shanghai, China. EverBridge Group's neuroscience business unit presented its comprehensive neurointerventional solutions at OCIN 2024, engaging in discussions on new concepts for stroke Treatment and opening a new chapter in interventional devices.



On October 25, the "Great Carotid Forum" on carotid artery stenosis intervention was co-chaired by Professor Wan Jieqing, Director of the Cerebrovascular Disease Center at Renji Hospital of Shanghai Jiao Tong University School of Medicine; Professor Cao Hui, Director of the Cerebrovascular Disease Treatment Center at Nanjing Brain Hospital of Nanjing Medical University; and Professor Gao Lianbo, Director of the Cerebrovascular Disease Treatment Center at the Fourth of Hospital of China Medical University. Professor Zhang Yong, Director of the Neurointerventional department at the of Hospital of Qingdao University, and Professor Ma Liang, Deputy Director of the Neurointerventional department at Hebei Provincial People's Hospital, delivered specialized presentations.



Professor Cao Hui, Nanjing Brain Hospital of Nanjing Medical University:
Carotid interventional devices have evolved from being entirely imported to a flourishing domestic market, particularly with the emergence of products tailored for carotid indications, such as embolic protection devices, carotid balloons, and carotid stents. The rise of domestic manufacturers has provided clinicians and patients with more accessible and trustworthy products.
Professor Zhang Yong, of Hospital of Qingdao University, on the Application of EverBridge Group's Carotid Products in Complex Carotid Artery Stenting procedure:

Although there is no unified definition for complex carotid artery lesions, and standards vary across centers, the Treatment is highly challenging and risky, significantly impacting patient prognosis. Professor Zhang shared two cases using EverBridge Group's products in complex carotid artery stenting, along with clinical insights on device usage.
Case 1: A patient with grade 3 hypertension and coronary heart disease presented with linear stenosis of the common carotid artery and occlusion of the internal carotid artery with ulceration. Implanting a distal protection device was challenging. The PathGuard embolic protection device demonstrated excellent deliverability, with the delivery sheath reaching wherever the microguidewire could. The device's soft structure ensured smooth placement and optimal wall apposition, effectively preventing distal thrombus escape while ensuring successful surgery.
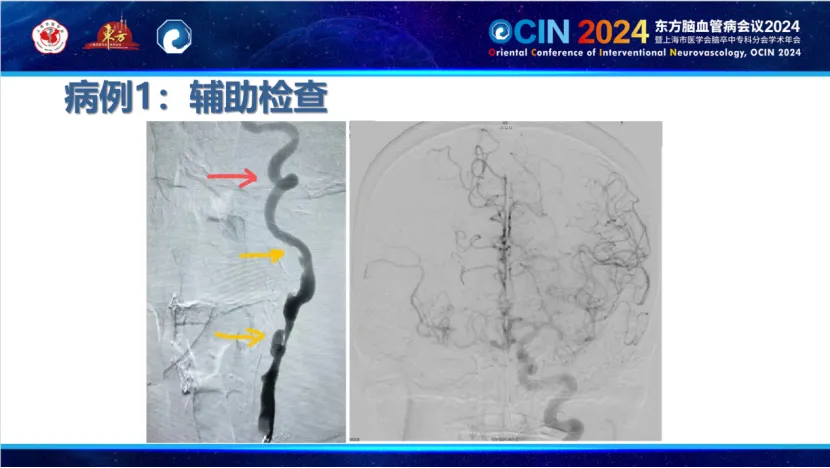
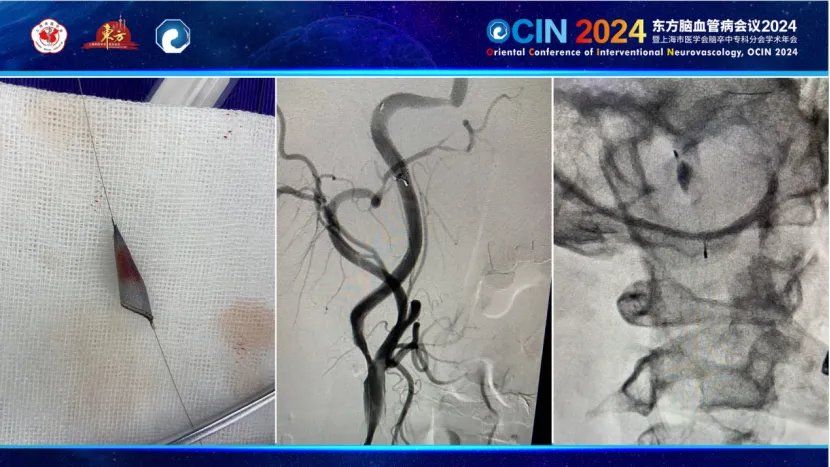
Case 2: A patient with hypertension and type 2 diabetes, experiencing dizziness for 7-8 years and left limb clumsiness for one month, was diagnosed with right internal carotid artery occlusion and middle cerebral artery visualization on MRA. The surgery utilized a carotid stent from PuGao Medical, currently under clinical study. The stent exhibited excellent visualization, facilitating positioning and real-time observation during deployment. Its superior wall apposition allowed smooth post-dilation and retrieval of the embolic protection device. The stent's moderate support reduced vagal reflexes and lowered long-term stenosis rates.
Professor Gao Lianbo, Fourth of Hospital of China Medical University, commented on the cases:
Building on embolic protection devices and dedicated carotid balloons, the introduction of a domestically produced carotid stent by EverBridge Group will further advance the development of a comprehensive domestic solution for carotid artery stenosis intervention. Domestic device innovation is not mere replication but involves continuous optimization and iteration based on independent R&D and production, addressing clinical pain points. Better and more user-friendly products are the driving force behind the evolution of domestic device solutions.
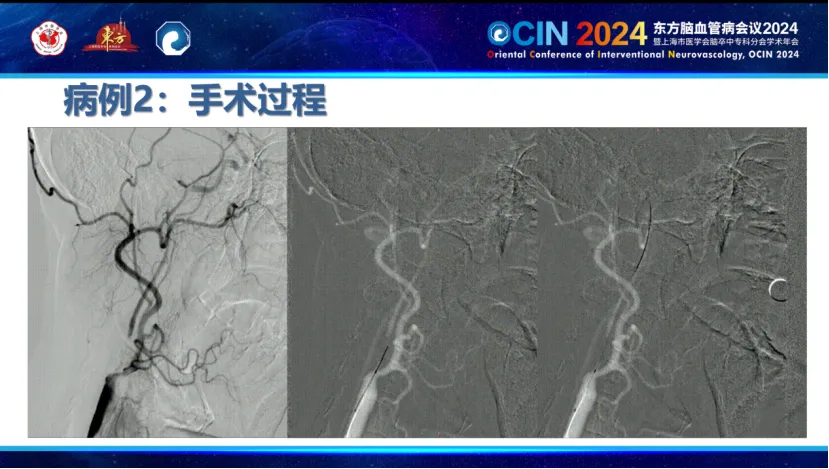
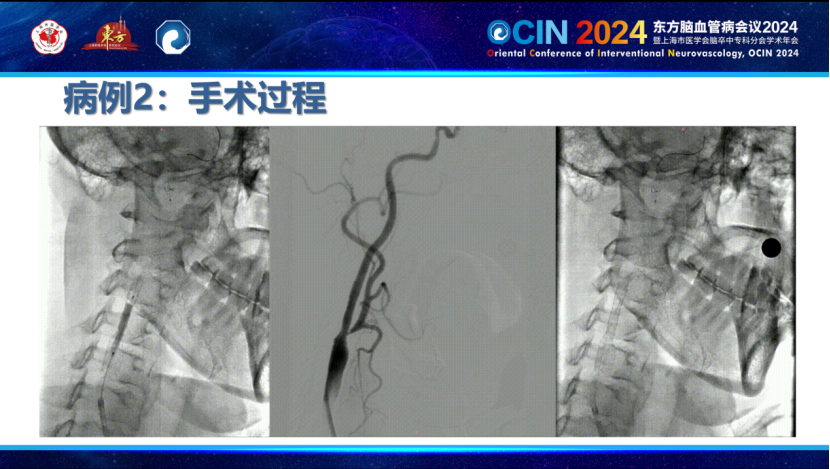
A Case of Staged Treatment for Carotid Near-Occlusion by Professor Ma Liang, Hebei Provincial People's Hospital:

A patient was admitted for cerebral infarction caused by severe stenosis of the right internal carotid artery (C1 segment). Due to near-occlusion of the right internal carotid artery and subacute cerebral infarction, with both old and new infarcts in the right middle cerebral artery territory, a staged Treatment plan was adopted: initial small balloon dilation during the acute phase, with stent placement as an option, followed by carotid artery stenting in the second phase. This approach allowed gradual improvement in cerebral blood flow, minimized impact on cerebral autoregulation, and enabled more precise selection of balloon or stent sizes.
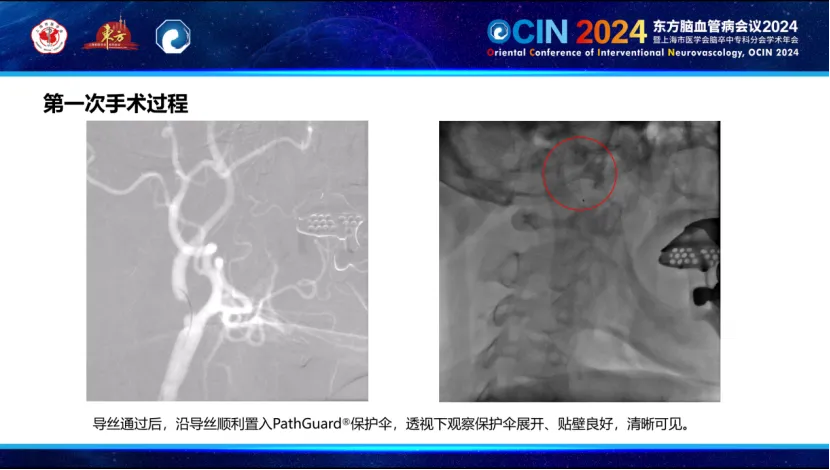
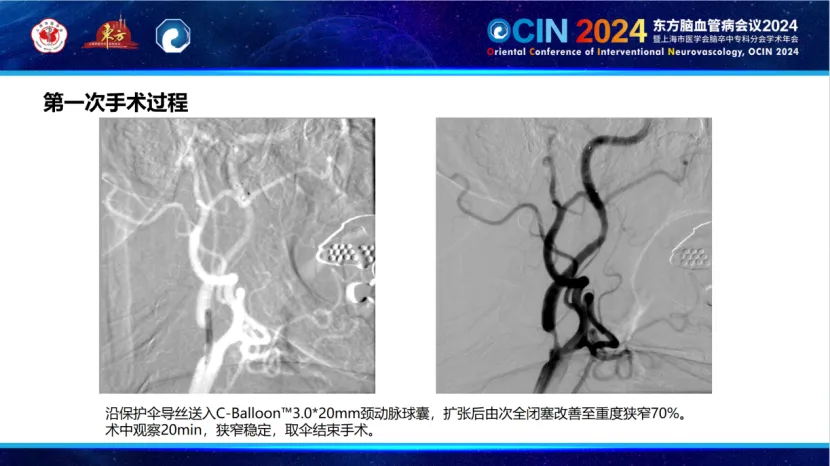
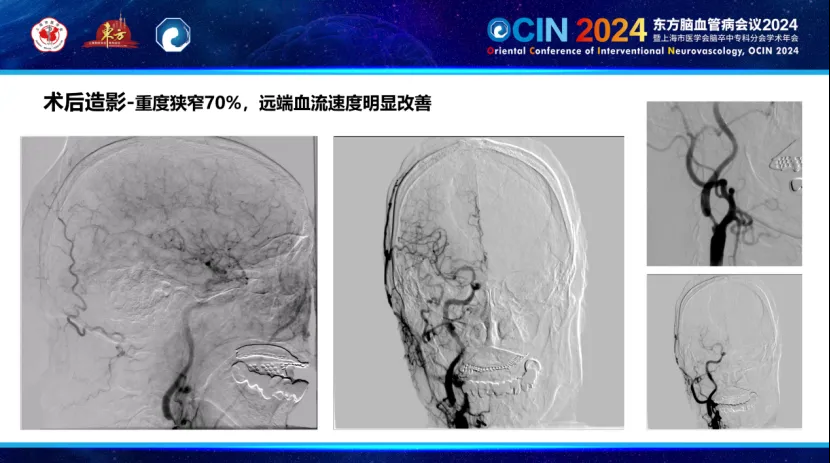
During the first phase, the Smartpioneer™ microguidewire demonstrated excellent torque control and support. The PathGuard embolic protection device successfully navigated the near-occlusion, achieving good wall apposition and clear visualization. The C-Balloon™ carotid balloon was then used for dilation, significantly improving distal blood flow on post-operative angiography.
Professor Wan Jieqing, Renji Hospital of Shanghai Jiao Tong University School of Medicine, commented on the case:
The advancement of neurointervention relies on the optimization and iteration of devices. The transition from fully imported to domestically produced devices was a significant leap. Further breakthroughs will come from meeting clinical needs. Effective integration of clinical experience with device R&D and production—through clinician-engineer collaboration—holds the key to future innovation. We look forward to more such collaborative projects, moving from clinical practice to R&D and back to clinical application, benefiting more patients.
EverBridge Group currently covers high-incidence chronic disease areas such as peripheral vascular, neuroscience, and integrated cancer diagnosis and Treatment. Leveraging six specialized R&D platforms—"multi-adaptation balloons," "full-function catheters," "cross-scenario stents," "bio-interface coatings," "high-performance guidewires," and "smart active devices"—along with a lean production center, the group has secured approvals for dozens of innovative products in its neuroscience business unit, offering a complete neurointerventional solution portfolio. Moving forward, EverBridge Group will continue to promote accessable and innovative neurointerventional solutions, driving high-quality and scalable development, and leading a new chapter in the innovation and implementation of neurointerventional devices.











